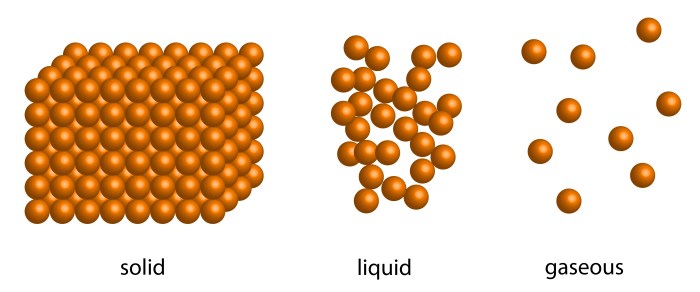Matter Crossword Puzzle Answer Key embarks on an enlightening journey into the fundamental nature of matter, its diverse forms, and its ubiquitous presence in our daily lives. Delving into the heart of scientific inquiry, this comprehensive guide unravels the mysteries of matter, empowering you with the knowledge to conquer any crossword puzzle challenge that comes your way.
As we embark on this exploration, we will dissect the defining characteristics of matter, unraveling the intricate tapestry of its physical and chemical properties. We will delve into the captivating world of elements, compounds, and mixtures, unraveling their unique compositions and structures.
Definition of Matter: Matter Crossword Puzzle Answer Key
Matter is anything that has mass and occupies space. It is the physical substance of the universe, and it exists in various forms and states.
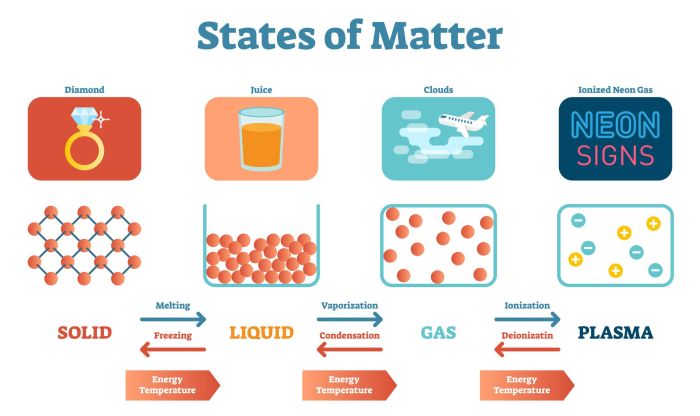
States of Matter
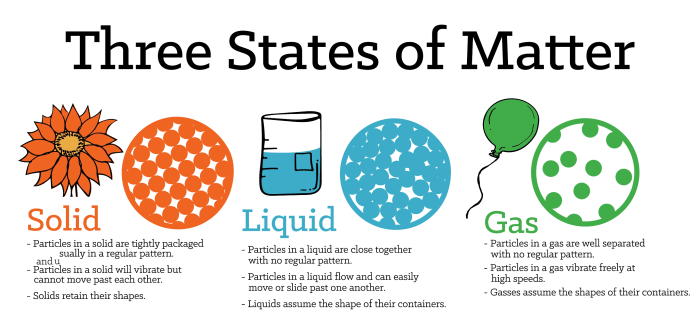
Matter can exist in three primary states:
- Solid:Definite shape and volume; particles tightly packed.
- Liquid:Definite volume but no definite shape; particles loosely packed.
- Gas:No definite shape or volume; particles widely dispersed.
Classification of Matter
Matter can be classified into three main categories:
- Elements:Pure substances that cannot be broken down into simpler substances by chemical means.
- Compounds:Substances composed of two or more elements chemically combined in a fixed ratio.
- Mixtures:Combinations of two or more substances that are not chemically combined.
Properties of Matter, Matter crossword puzzle answer key
Matter possesses various physical and chemical properties that help identify and distinguish it:
Physical Properties
- Density:Mass per unit volume.
- Melting point:Temperature at which a solid changes to a liquid.
- Boiling point:Temperature at which a liquid changes to a gas.
- Color:Wavelength of light reflected by the substance.
Chemical Properties
- Reactivity:Ability of a substance to undergo chemical reactions.
- Flammability:Ability of a substance to burn.
- Acidity:Ability of a substance to release hydrogen ions in water.
- Solubility:Ability of a substance to dissolve in a solvent.
Changes in Matter
Matter undergoes various types of changes:
Physical Changes
- Changes in state:Melting, freezing, boiling, condensing.
- Changes in shape:Bending, stretching, cutting.
- Changes in size:Expansion, contraction.
Chemical Changes
- Chemical reactions:Formation of new substances with different properties.
- Combustion:Reaction with oxygen, releasing heat and light.
- Decomposition:Breaking down of a substance into simpler substances.
Matter in Everyday Life
Matter is essential in our daily lives:
- Food:Provides nutrients and energy.
- Clothing:Protects and insulates.
- Buildings:Provide shelter and protection.
- Transportation:Vehicles and fuel.
- Technology:Electronic devices, computers, medical equipment.
Q&A
What is the definition of matter?
Matter is anything that has mass and takes up space.
What are the three states of matter?
The three states of matter are solid, liquid, and gas.
What is the difference between an element, a compound, and a mixture?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. A compound is a pure substance that is made up of two or more elements that are chemically combined. A mixture is a combination of two or more substances that are not chemically combined.